Mountain View, Calif. – Cordance Medical, a development-stage medical device company, announces that its NeuroAccess™ device has demonstrated a completely non-invasive and targeted blood-brain barrier (BBB) opening in pre-clinical studies involving large animals.
“The ability to safely and accurately open up targeted regions of the brain non-invasively is a key milestone in the development of a patient-centric device,” said Bhaskar Ramamurthy, Ph.D., co-founder & CTO at Cordance Medical. “We plan to utilize the NeuroAccess™ device in human studies this year.” The NeuroAccess™ device is a focused ultrasound system that opens the BBB precisely to physician-directed brain regions in a simple, non-invasive 30-minute outpatient clinic procedure. The NeuroAccess™ device is being validated pre-clinically with planned trials commencing in the summer of 2023.
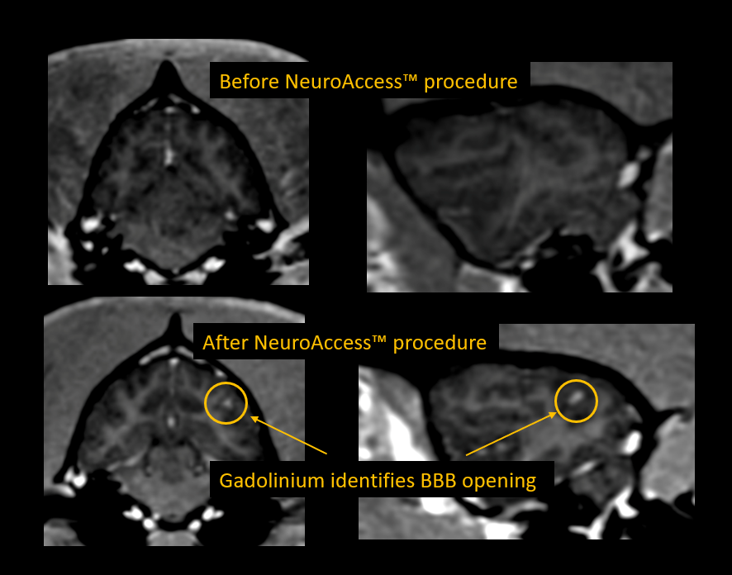
Safe & effective opening of the BBB: Images on top are MR images of a canine after gadolinium contrast, showing an intact BBB. Images on the bottom represent MR images after the use of the NeuroAccess™ device, showing gadolinium penetrating the BBB and staining portions of the brain. The image on the right shows the histology of brain tissue with Evans Blue staining of the opened BBB region.
